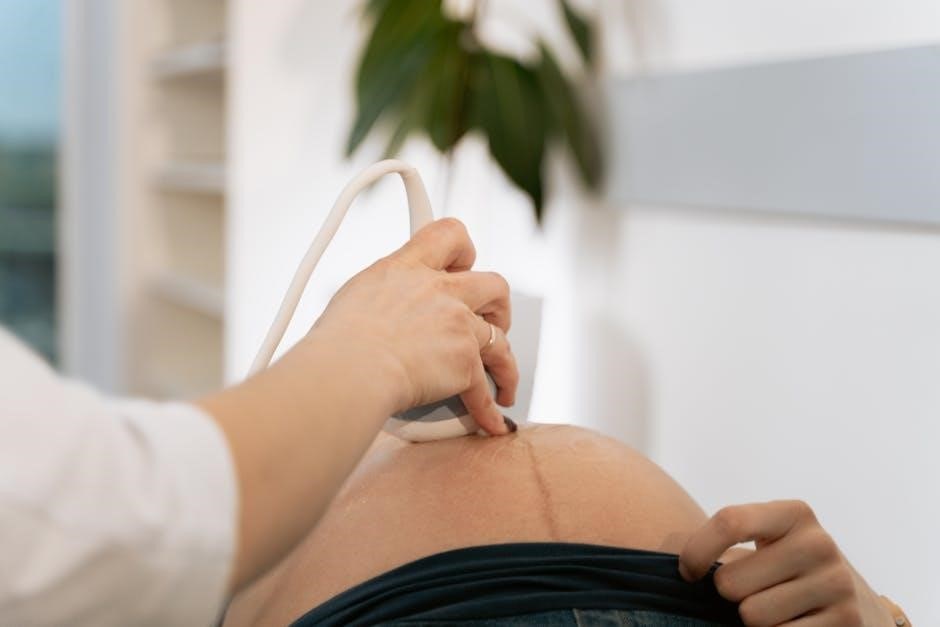
Ultrasound Guided Thyroid Biopsy CPT Codes: A Comprehensive Guide
Understanding the correct CPT codes for ultrasound-guided thyroid biopsies is crucial for accurate billing and coding practices; 10005 and 10006 are frequently utilized.
Thyroid biopsies are essential diagnostic procedures used to investigate abnormalities within the thyroid gland, often prompted by palpable nodules or findings on imaging studies like ultrasound. These biopsies help determine whether a nodule is benign or malignant, guiding subsequent treatment decisions. The most common technique is fine needle aspiration (FNA), frequently performed under ultrasound guidance for precision and accuracy.
Accurate coding, utilizing CPT codes like 10005 for the initial lesion and 10006 for each additional lesion biopsied during the same session, is paramount for proper reimbursement. Understanding the nuances of these codes, along with appropriate modifiers, ensures compliant billing practices. The procedure involves extracting a small sample of cells from the thyroid nodule for pathological examination, providing critical information for patient care.
What is Ultrasound Guided Thyroid Biopsy?
Ultrasound-guided thyroid biopsy is a minimally invasive procedure where a fine needle is used to extract cells from a thyroid nodule, aided by real-time ultrasound imaging. This imaging allows the physician to precisely visualize the nodule and guide the needle to the target area, increasing diagnostic accuracy and minimizing discomfort for the patient. CPT codes 10005 (first lesion) and 10006 (each additional lesion) are specifically used to report this procedure.
The ultrasound not only guides the needle but also helps assess nodule characteristics – size, shape, and internal features – which are crucial for determining the need for a biopsy. Proper documentation supporting the use of these CPT codes, including ultrasound findings, is essential for accurate billing and avoiding claim denials.
The Role of Ultrasound in Thyroid Nodule Evaluation
Ultrasound is the primary imaging modality for evaluating thyroid nodules, providing detailed information about their size, composition, and characteristics. This assessment is critical in determining which nodules require further investigation, such as a fine needle aspiration (FNA) biopsy, coded with CPT codes 10005 or 10006. Ultrasound helps differentiate between benign and potentially malignant nodules, guiding clinical decision-making.
The American College of Radiology (ACR) TI-RADS system utilizes ultrasound features to categorize nodules based on their risk of malignancy. This standardized approach aids in biopsy recommendations and ensures consistent reporting. Accurate ultrasound interpretation is paramount for appropriate CPT code selection and avoiding unnecessary procedures.
CPT Codes for Thyroid Biopsy Procedures
Specific CPT codes, like 10005 for the initial lesion and 10006 for each additional one, are essential for billing ultrasound-guided thyroid FNA biopsies correctly.
CPT Code 10005: Fine Needle Aspiration Biopsy, First Lesion
CPT code 10005 represents the fine needle aspiration (FNA) biopsy of a single, initial thyroid lesion, performed under ultrasound guidance. This code encompasses the entire procedure, including the ultrasound imaging utilized for precise needle placement and visualization. It’s crucial to remember that this code is specifically for the first lesion biopsied during a single session.
Proper documentation is paramount when utilizing this code. The medical record should clearly indicate the use of ultrasound guidance, the specific location of the biopsied nodule within the thyroid gland, and the technique employed during the FNA. Accurate coding ensures appropriate reimbursement and reflects the skilled intervention performed. Furthermore, understanding the nuances of this code prevents potential billing errors and audits.
This code is frequently used in conjunction with other codes representing the ultrasound examination itself, but careful attention must be paid to avoid improper bundling.
CPT Code 10006: Each Additional Lesion
CPT code 10006 is utilized to report each additional thyroid lesion biopsied during the same session as the initial lesion coded with 10005. This means that after the first FNA biopsy is performed and coded as 10005, any subsequent biopsies of separate nodules within the thyroid gland require the use of 10006 for each one.
Like 10005, meticulous documentation is essential. The medical record must clearly delineate each distinct lesion biopsied, its location, and the ultrasound guidance used. Using 10006 correctly reflects the increased time, skill, and resources required for multiple biopsies.
It’s vital to avoid misusing this code; it’s not for repeat biopsies of the same lesion, but for distinctly separate nodules. Accurate application of 10006 ensures appropriate billing and avoids potential compliance issues.
Understanding Modifiers for Thyroid Biopsy CPT Codes
Modifiers play a crucial role in accurately representing the circumstances of an ultrasound-guided thyroid biopsy. While not always required, they can be essential for proper reimbursement. For instance, modifier -59 may be appended to 10006 if multiple lesions are biopsied during the same session, but are not necessarily distinct or separate enough to warrant separate coding without the modifier.
Furthermore, if the procedure is performed under unusual circumstances requiring significantly more effort or time, modifiers like -22 could be considered. Accurate documentation supporting the use of any modifier is paramount.
Incorrect modifier usage can lead to claim denials, so a thorough understanding of coding guidelines and payer policies is vital for healthcare professionals and billing departments.

Indications for Ultrasound Guided Thyroid Biopsy
Biopsies are indicated for thyroid nodules exhibiting suspicious ultrasound features, specific size thresholds, or concerning patient history and risk factors.
Thyroid Nodules: Size and Characteristics
Determining whether a thyroid nodule requires biopsy often hinges on its size and specific characteristics observed during ultrasound evaluation. Generally, nodules exceeding 1 centimeter in diameter warrant consideration for fine needle aspiration (FNA). However, smaller nodules—even those less than 1 cm—may also be biopsied if they display high-risk ultrasound features.
These features include microcalcifications, irregular margins, a taller-than-wide shape, and evidence of extrathyroidal extension. The American Thyroid Association (ATA) guidelines provide detailed recommendations based on nodule size and sonographic risk stratification. Accurate measurement and detailed description of nodule characteristics are essential for appropriate biopsy decisions and correct CPT code selection.
Suspicious Ultrasound Features
Identifying suspicious features on thyroid ultrasound is paramount in guiding biopsy decisions and selecting the appropriate CPT code. Key indicators include the presence of microcalcifications – tiny, punctate echoes – which are often associated with papillary thyroid cancer. Irregular nodule margins, a shape taller than wide, and marked hypoechogenicity (darkness on the ultrasound) also raise concern.
Furthermore, evidence of central vascularity, particularly in nodules lacking other benign features, can suggest malignancy. The Thyroid Imaging Reporting and Data System (TI-RADS) categorizes nodules based on these characteristics, providing standardized risk assessment. Documenting these features meticulously ensures accurate coding and appropriate clinical management following the ultrasound-guided FNA biopsy.
Patient History and Risk Factors
Evaluating a patient’s history and risk factors is essential before considering an ultrasound-guided thyroid biopsy and selecting the correct CPT code. A history of prior head and neck radiation exposure significantly increases cancer risk, warranting closer scrutiny. Family history of thyroid cancer also elevates concern, prompting consideration for biopsy even with less suspicious ultrasound findings.
Rapid nodule growth, hoarseness, or difficulty swallowing are concerning symptoms that necessitate investigation. Age is a factor; while cancer can occur at any age, it’s more common in younger individuals. Careful documentation of these factors, alongside ultrasound characteristics, supports appropriate biopsy decisions and accurate procedural coding, like utilizing CPT code 10005 or 10006.
The Biopsy Procedure: A Step-by-Step Overview
The procedure involves ultrasound guidance for precise needle placement, sample collection, and proper handling, all documented for accurate CPT code assignment.
Patient Preparation
Prior to the ultrasound-guided thyroid biopsy, patients typically require minimal preparation, though specific instructions may vary by facility. Generally, patients can continue taking their regular medications unless otherwise directed by their physician. It’s important to inform the doctor about any blood thinners or aspirin use, as these might increase bleeding risk.
Patients are usually asked to remove jewelry and clothing from the neck area. A brief medical history review, including allergies and prior thyroid conditions, is standard. The procedure and potential risks, alongside the relevant CPT codes (10005, 10006) for billing, are explained. Ensuring patient understanding and obtaining informed consent are paramount before proceeding with the biopsy.
Ultrasound Guidance and Needle Placement
During the ultrasound-guided thyroid biopsy, the patient lies supine with their neck extended. A skilled sonographer utilizes real-time ultrasound imaging to visualize the thyroid nodule, guiding precise needle placement. A thin, sterile needle, attached to a syringe, is advanced through the skin and into the target nodule.
The ultrasound ensures accurate targeting, minimizing discomfort and maximizing sample acquisition. The procedure, often coded with CPT codes 10005 or 10006, is performed under sterile conditions. Gentle pressure is applied during needle insertion. The sonographer continuously monitors the needle’s position, confirming it’s within the nodule before aspirating cells for analysis.
Sample Collection and Handling
Following successful needle placement, gentle suction is applied to the syringe, collecting cells from the thyroid nodule. Multiple passes may be performed, rotating the needle within the nodule to obtain a representative sample – procedures often billed using CPT codes 10005 and 10006.
The collected sample is then expelled onto glass slides for immediate fixation in alcohol, preserving cellular morphology. Additional material may be placed in a fluid-based cytology medium. Proper labeling is critical, including patient identifiers and the nodule’s location. These slides are promptly sent to a cytopathologist for microscopic examination, aiding in diagnosis and treatment planning.

Accuracy and Limitations of Ultrasound Guided Thyroid Biopsy
While generally reliable, ultrasound-guided thyroid biopsies (CPT 10005/10006) can yield nondiagnostic results, influenced by factors like nodule size and cellularity.
Factors Affecting Nondiagnostic Results
Several elements can contribute to nondiagnostic results during ultrasound-guided fine needle aspiration (FNA) biopsies, coded as CPT 10005 or 10006. Nodule size, particularly very small nodules, can make accurate sampling challenging. Cystic nodules, containing fluid, often yield insufficient cellular material for analysis. The experience and skill of the operator performing the biopsy significantly impact success rates; proper needle placement is vital.
Additionally, inadequate sample collection, due to insufficient passes or improper technique, is a common cause. Blood contamination within the sample can obscure cellular details, hindering accurate interpretation. Patient-related factors, such as anti-coagulant medication use, may increase the risk of bleeding and dilute the sample. Finally, the specific characteristics of the nodule itself, including fibrosis or calcification, can impede needle access and cellular retrieval.
The Role of TI-RADS in Biopsy Decision Making
The American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS) plays a pivotal role in determining the necessity of an ultrasound-guided thyroid biopsy, often coded with CPT codes 10005 or 10006. TI-RADS categorizes nodules based on ultrasound characteristics, assigning a risk stratification from TR1 to TR5.
Nodules classified as TR3 have an intermediate suspicion level, and biopsy consideration is individualized; TR4 and TR5 nodules demonstrate a higher risk of malignancy, strongly recommending FNA for diagnosis. Utilizing TI-RADS helps standardize reporting, reduce inter-observer variability, and minimize unnecessary biopsies of benign nodules. This standardized approach ensures appropriate resource allocation and improves patient care by focusing biopsy efforts on nodules with a genuine concern for cancer.
AI and its Impact on Reducing Unnecessary Biopsies
Artificial intelligence (AI) is increasingly impacting thyroid nodule evaluation, potentially reducing the number of ultrasound-guided biopsies – procedures often coded with CPT codes 10005 and 10006. AI algorithms analyze ultrasound images to identify features indicative of malignancy with high accuracy.
By accurately classifying low-risk nodules, AI can help clinicians avoid unnecessary FNAs, decreasing patient anxiety and healthcare costs. These systems can differentiate benign nodules from those requiring further investigation, leading to more informed biopsy decisions. The integration of AI doesn’t replace the radiologist, but serves as a valuable tool to enhance diagnostic precision and optimize the use of resources, ultimately improving patient outcomes.
Billing and Coding Considerations
Accurate documentation is vital when billing for ultrasound-guided thyroid biopsies (CPT 10005, 10006) to avoid denials and ensure proper reimbursement.
Proper Documentation for Accurate Coding
Detailed documentation is paramount for justifying the use of CPT codes 10005 and 10006 during ultrasound-guided thyroid biopsies. The medical record should clearly state the number of lesions biopsied, confirming whether it was the initial lesion (10005) or additional ones (10006).
Specifically, document the nodule size, location, and any suspicious ultrasound characteristics that prompted the biopsy. Include the technique used, the needle gauge, and any complications encountered. Precise reporting of the procedure, including real-time ultrasound guidance, is essential.
Furthermore, the documentation must support medical necessity, aligning with established guidelines like TI-RADS. A lack of thorough documentation can lead to claim denials or audits, emphasizing the importance of comprehensive record-keeping.
Avoiding Common Coding Errors
A frequent error involves incorrectly applying CPT codes for ultrasound-guided thyroid biopsies. Avoid combining codes for the thyroid biopsy (10005/10006) with separate codes for a general neck ultrasound examination; these are often bundled.
Another mistake is miscounting the number of biopsied lesions, leading to incorrect use of 10005 (first lesion) and 10006 (each additional lesion). Ensure accurate documentation supports the number of lesions targeted.
Furthermore, failing to document medical necessity, such as suspicious ultrasound features or TI-RADS categorization, can result in claim denials. Always verify payer-specific guidelines and stay updated on coding changes to prevent errors.
Bundling and Unbundling Issues
The performance of an ultrasound-guided thyroid biopsy often presents bundling challenges. Typically, the ultrasound guidance is considered integral to the biopsy procedure and is included within the CPT codes 10005 or 10006, not separately billable.
However, if a comprehensive neck ultrasound is performed prior to, and independently from, the targeted biopsy, it may be separately reportable, depending on documentation and medical necessity.
Unbundling – inappropriately billing for both the complete ultrasound and the biopsy guidance – is a common audit trigger. Accurate documentation demonstrating distinct services is vital. Payer policies vary, so verification is essential to avoid claim denials.

Thyroid Imaging Reporting and Data System (TI-RADS)
TI-RADS categorizes thyroid nodules based on ultrasound features, guiding biopsy recommendations and influencing the necessity of CPT codes 10005/10006.
TI-RADS Categories and Biopsy Recommendations
The American College of Radiology (ACR) TI-RADS system classifies thyroid nodules into six categories (TR1-TR6) based on ultrasound characteristics. Lower categories (TR1-TR3) generally indicate a low risk of malignancy and often warrant observation with repeat ultrasound, potentially avoiding immediate biopsy and associated CPT codes like 10005 or 10006.
However, nodules in higher categories (TR4-TR6) have an increased suspicion for cancer. TR4 nodules have intermediate suspicion and biopsy recommendations vary based on size; larger nodules generally require fine needle aspiration (FNA) utilizing CPT code 10005 for the first lesion and 10006 for each additional lesion biopsied during the same session. TR5 and TR6 nodules have high and very high suspicion, respectively, strongly recommending biopsy with the same CPT coding guidelines.
Proper application of TI-RADS helps standardize risk assessment and guides appropriate clinical management, including the decision to proceed with an ultrasound-guided FNA biopsy.
ACR TI-RADS: A Standardized Approach
The ACR TI-RADS provides a standardized framework for reporting thyroid nodule ultrasound findings, enhancing communication and reducing inter-observer variability. This system utilizes specific sonographic features – composition, echogenicity, shape, margins, and presence of microcalcifications – to assign a risk stratification. Consistent application of TI-RADS directly influences biopsy decisions and, consequently, the utilization of CPT codes such as 10005 (initial lesion biopsy) and 10006 (additional lesion biopsy).
By establishing clear criteria, ACR TI-RADS minimizes unnecessary biopsies for benign nodules, potentially reducing healthcare costs and patient anxiety. Conversely, it ensures timely evaluation of suspicious nodules, facilitating prompt diagnosis and treatment. Accurate TI-RADS assessment is paramount for appropriate CPT code selection and optimal patient care.
The Future of TI-RADS
Ongoing research focuses on refining TI-RADS to further improve its predictive accuracy and minimize indeterminate results, impacting ultrasound-guided thyroid biopsy utilization. Integration of artificial intelligence (AI) promises automated nodule characterization, potentially streamlining TI-RADS assessment and reducing unnecessary biopsies, thus influencing CPT code application (10005/10006).
Future iterations may incorporate molecular markers and radiomics data to enhance risk stratification, leading to more precise biopsy recommendations. The development of standardized training programs for TI-RADS interpretation is also crucial. Ultimately, advancements in TI-RADS aim to optimize the balance between detecting clinically significant cancers and avoiding overtreatment, directly affecting the appropriate use of CPT codes for thyroid biopsies.
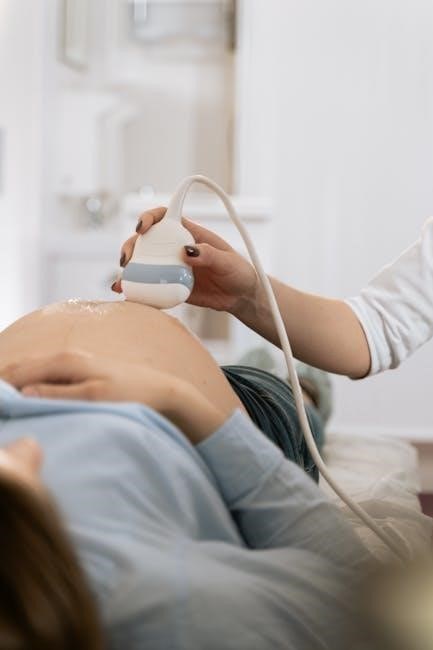
Alternatives to Biopsy
Active surveillance and molecular testing offer alternatives, potentially decreasing the need for ultrasound-guided biopsies and associated CPT codes like 10005 or 10006.
Active Surveillance for Low-Risk Nodules
For thyroid nodules assessed as low-risk based on ultrasound characteristics and TI-RADS categorization, active surveillance presents a viable alternative to immediate biopsy and associated CPT coding. This approach involves regular, typically six to twelve-month, follow-up ultrasound examinations to monitor nodule size and features.
Avoiding upfront biopsy—and thus, the use of codes like 10005 or 10006—reduces patient anxiety and potential complications. If the nodule remains stable or decreases in size, continued surveillance is appropriate. However, any significant growth or development of suspicious features necessitates a biopsy, triggering the need for accurate CPT code assignment at that time. This strategy minimizes unnecessary procedures and associated costs.
Molecular Testing as a Complement to Biopsy
Molecular testing is increasingly utilized alongside ultrasound-guided thyroid biopsies, potentially refining risk stratification and reducing indeterminate biopsy results. While not directly coded with CPT codes 10005 or 10006, these tests often follow an initial biopsy. They analyze genetic markers within the nodule sample to assess the likelihood of malignancy.
These tests can help clarify indeterminate cytology results, guiding clinical decisions and potentially avoiding unnecessary repeat biopsies or surgery. The results influence patient management, but the initial ultrasound-guided biopsy remains the primary diagnostic procedure requiring specific CPT coding. Molecular testing adds a layer of precision, complementing—not replacing—traditional diagnostic pathways.
Repeat Ultrasound Monitoring
Repeat ultrasound monitoring serves as an alternative to immediate biopsy for small, low-risk thyroid nodules, and doesn’t directly involve CPT codes 10005 or 10006. This approach involves serial imaging to track nodule growth and characteristics over time. If a nodule exhibits concerning changes – increased size or suspicious features – an ultrasound-guided biopsy then becomes necessary, triggering the appropriate CPT coding.
The interval between scans varies based on initial risk assessment. This strategy avoids unnecessary invasive procedures and associated costs; While monitoring itself isn’t coded with biopsy-specific CPT codes, the eventual biopsy, if required, will be billed accordingly, reflecting the initial assessment and subsequent intervention.

Post-Biopsy Care and Follow-Up
Post-biopsy care focuses on managing discomfort; CPT codes 10005/10006 cover the procedure, but follow-up doesn’t have specific billing codes.
Managing Discomfort and Bleeding
Following an ultrasound-guided thyroid biopsy, patients may experience mild discomfort or a small amount of bruising at the puncture site; this is generally self-limiting. Applying a cold compress can help minimize swelling and alleviate any pain. While significant bleeding is rare, a small bandage should be applied to the area.
CPT codes 10005 and 10006 specifically cover the biopsy procedure itself, and do not include post-procedure management. Patients should be advised to avoid strenuous activity for 24 hours. If excessive bleeding, signs of infection (fever, increased pain, redness), or difficulty breathing occur, immediate medical attention is necessary. Over-the-counter pain relievers, like acetaminophen, can typically manage any residual discomfort.
Interpreting Biopsy Results
The results of an ultrasound-guided thyroid biopsy, billed using CPT codes 10005 or 10006, are typically reported as benign, suspicious, or malignant. A benign result indicates no cancer is present, but follow-up ultrasounds are often recommended. Suspicious results suggest the possibility of cancer and may warrant repeat biopsy or molecular testing. A malignant result confirms cancer, requiring further evaluation and treatment planning.
Pathologists classify biopsies using the Bethesda System, providing a standardized reporting framework. Understanding these classifications is crucial for appropriate patient management. It’s important to remember that a non-diagnostic result doesn’t necessarily indicate cancer, but necessitates repeating the biopsy procedure to obtain a sufficient sample for accurate analysis.
Long-Term Follow-Up Recommendations
Following an ultrasound-guided thyroid biopsy – procedures coded with 10005 or 10006 – long-term follow-up depends heavily on the biopsy results. Benign nodules typically require periodic ultrasound monitoring, often annually or every two years, to assess for growth or changes. Suspicious or indeterminate results may necessitate closer surveillance, potentially including repeat biopsies or molecular testing.
Patients with confirmed thyroid cancer require ongoing monitoring for recurrence, including regular physical exams, thyroid function tests, and potentially, further imaging. The frequency of follow-up is tailored to the cancer stage and treatment received. Adherence to these recommendations is vital for early detection and management of any potential issues.